Common reference intervals and quality
specifications
Reference intervals are established for the nine
proteins according to age and sex (and use of
estrogens) based on more than 500 healthy indi–
viduals in Denmark and campared to a Finnish
population. The results will be given to the
project-participants and published elsewhere as
soon as the IFCC protein calibrator has been re–
leased and the IFCC-accepted-values transfer–
red to the Nordie protein calibrator (see below).
The goals for achiving these reference inter–
vals were established for the project based on
published reference intervals according to the
principles for sharing common reference inter–
vals (7,8). Assuming negligible analytical varia–
tion (imprecision) the specifications for maxi–
mum analytical bias were:
• lgM and Haptoglobin- ± 120Jo
e
IgA- ± 10%
• lgG and Orosomucoid - ± 5%
• Prealbumin and a-Proteinase inhibitor
(a1-Antitrypsin)- ± 4%.
• Transferrio - ±
+
3%
e
Albumin-± 2%.
These specifications for maximum allowable
bias were used in the project, but will be actjust–
ed when common reference intervals are
released.
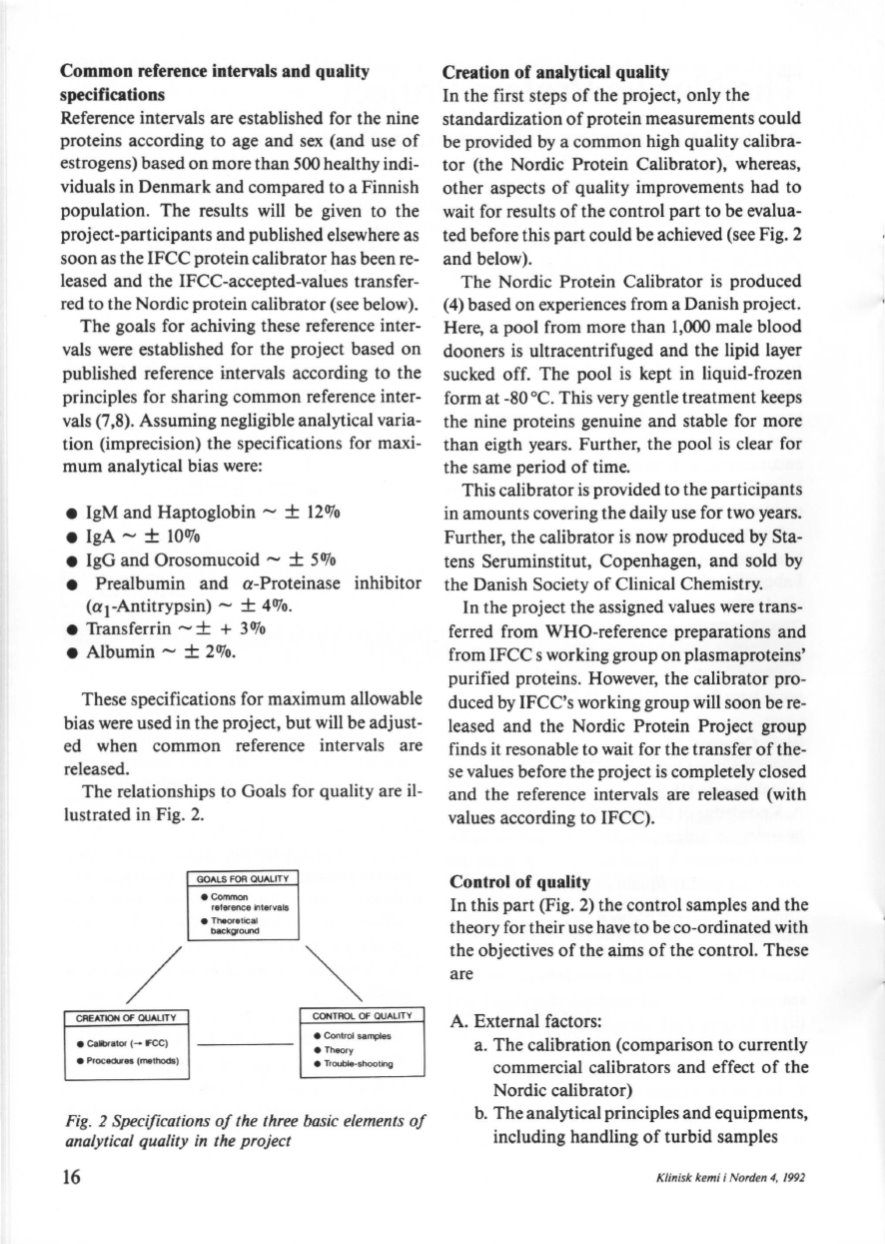
The relationships to Goals for quality are
il–
lustrated in Fig. 2.
GREATlON OF OUALITY
e
Galibrator (- FCC)
e
Procedlxes
{methods)
GOALS
FOR
QUALITY
e Common
reference intarvals
e
Theoretical
backgrot.nd
CONTROL OF QUALITY
e Control samplas
e
Theory
e
Troubkt·Shooting
Fig. 2 Specifications of the three basic elements of
analytical qua/ity in the project
16
Creation of analytical quality
In the first steps of the project, only the
standardization of protein measurements could
be provided by a common high quality calibra–
tor (the Nordie Protein Calibrator), whereas,
other aspects of quality improvements bad to
wait for results of the control part to be evalua–
ted before this part could be achieved (see Fig. 2
and below).
The Nordie Protein Calibrator is produced
(4) based on experiences from a Danish project.
Here, a pool from more than 1,000 male blood
doaners is ultracentrifuged and the lipid layer
sucked off. The pool is kept in liquid-frozen
form at -80 °C. This very gentie treatment keeps
the nine proteins genuine and stable for more
than eigth years. Further, the pool is clear for
the same period of time.
This calibrator is provided to the partidpants
in amounts covering the daily use for two years.
Further, the calibrator is now produced by Sta–
tens Seruminstitut, Copenhagen, and sold by
the Danish Society of Clinical Chemistry.
In the project the assigned values were trans–
ferred from WHO-reference preparations and
from IFCC s working group on plasmaproteins'
purified proteins. However, the calibrator pro–
duced by IFCC's working group will soon be re–
leased and the Nordie Protein Project group
finds it resonable to wait for the transfer of the–
se values before the project is completely ciased
and the reference intervals are released (with
values according to IFCC).
Control of quality
In this part (Fig. 2) the control samples and the
theory for their use have to be co-ordinated with
the objectives of the aims of the contro
l.
These
are
A. Externat factors:
a. The calibration (comparison to currently
commercial calibrators and effect of the
Nordie calibrator)
b. The analytical principles and equipments,
including handling of turbid samples
Klinisk kemi
i
Norden 4, 1992


