Klinisk Biokemi i Norden · 1 2017
| 9
Verifying bias and imprecision
Measurement
bias
is a quantitative concept - the
“closeness of agreement between the average of an
infinite number of replicate measured quantity values
and a reference quantity value” (12).
Trueness
is the
corresponding qualitative concept for bias.
Measurement
precision
is a qualitative concept
- the “closeness between indications or measured
quantity values obtained by replicate measurements
on the same or similar objects under the specified
conditions of measurement” (12). The quantitative
expression of precision is
imprecision
- the standard
deviation (SD) or the relative standard deviation (CV/
CV %) of a method.
Method A can be qualitatively expressed to be more
or less precise than method B. But when you need
to quantify precision, we measure its opposite – the
imprecision – by repeated measurements of control-
or patient samples.
Measurement
accuracy
is a qualitative concept
describing the “closeness of agreement between a
measured quantity value and a true quantity value
of a measurand” (12). It encompasses both systematic
and random error components.
Verification practices have been established over
time and are frequently influenced by accredita-
tion- and certification authorities. There is by now a
general agreement on what is required (1, 9-11) and
supported by guidelines including CLSI EP15 (3) and
EP9(20). The European IVD directive (19, 21) requi-
res that the in vitro diagnostic device in the hands
of its end users must achieve the performance stated
by the manufacturer including analytical sensitivity,
limits of detection, diagnostic sensitivity, analytical
specificity, diagnostic specificity, accuracy, repeata-
bility, reproducibility and control of known relevant
interferences. In practice user verification is usually
restricted to comparison of methods experiments to
establish bias, replication experiments to establish
imprecision and a linearity check to determine the
reportable range and sometimes analysing reference
samples to verify the reference interval.
Estimating bias
At least 20 natural patient samples having as wide
concentration range as possible are commonly mea-
sured using both the method being replaced and the
new method. Stable materials for internal quality
control with known expected concentrations are also
measured to estimate imprecision and preferably also
appropriate reference materials as independent mea-
sures of bias. Larger number of samples than 20 may,
however, be required if minute differences between
the old and the new method need to be estimated.
The results of the measurements are summari-
zed using bias- Bland-Altman plot and orthogonal/
Deming regression as described below.
Estimating imprecision
Suitable stable materials for internal quality control
are measured at two concentration levels in at least 2
replicates for at least 5 consecutive days for estimating
imprecision and for establishing initial control limits
for the internal quality control procedures.
Summarizing the results
Orthogonal linear regression (22, 23), bias-plot (24, 25)
and analysis of variance (26, 27) techniques are used
to determine bias, imprecision, matrix effects etc. An
example is given
ysis of
20 patient samp
stems.
SigmaPlot 12.5 (
/
) was
used for the pur
alyse-
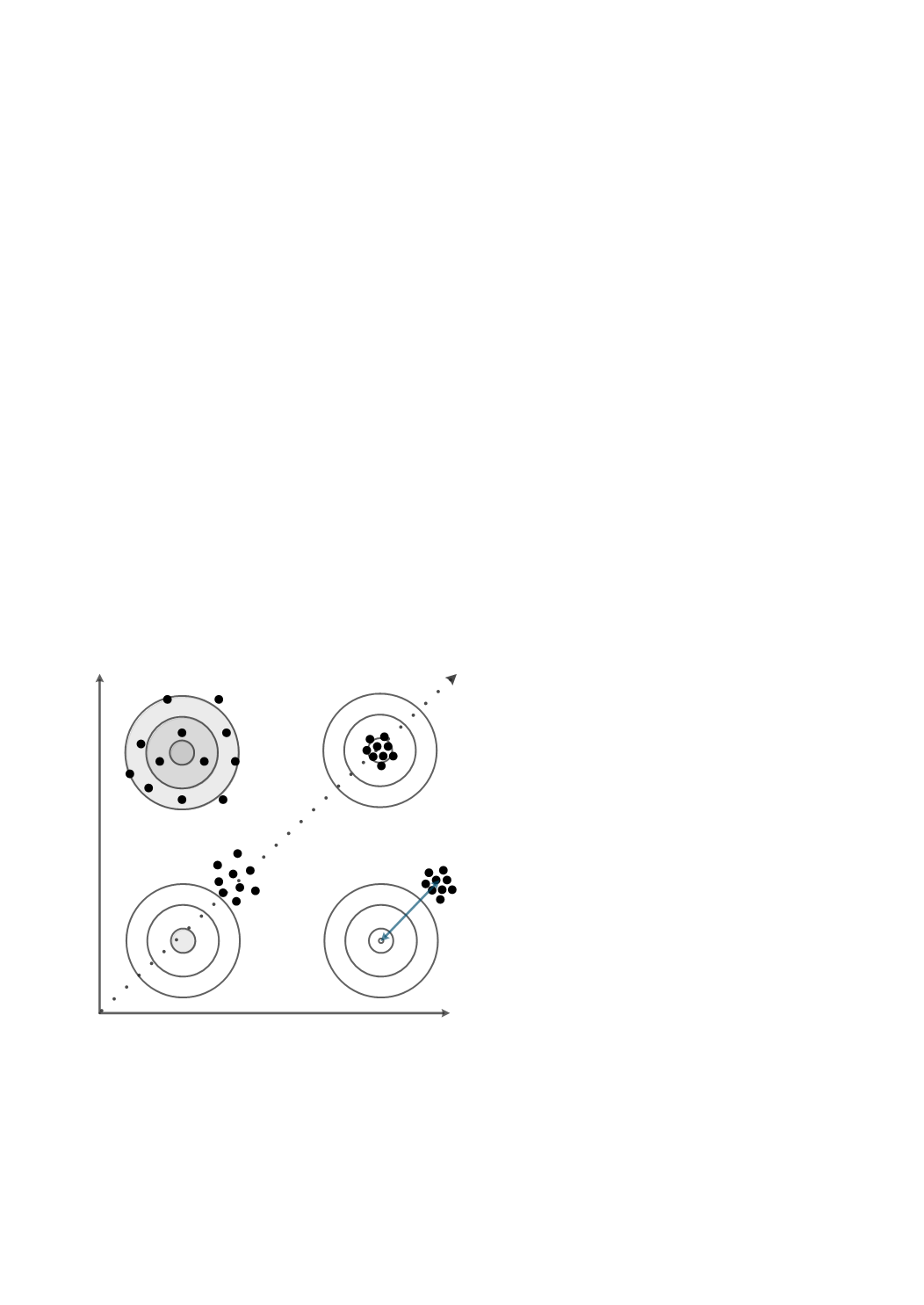
Figure 1:
A graphical illustration (using target analogy) of
the qualitative concepts precision and trueness and their
combination accuracy. Bias is decreased by increased tru-
eness and precision improved by decreasing imprecision.
Accuracy can be improved by improving precision or tru-
eness or both.
x
Improved precision
Improved trueness
Improved
accur acy
B ias


