14 |
Klinisk Biokemi i Norden · 1 2017
rate of laboratories, and is not aware of any guidelines
or standards for the purpose. It is, however, evident
that the sensitivity, specificity, predictive values etc.
of measurement results produced by a conglomerate
of laboratories may differ from those found during
the original full diagnostic validation because the
overall measurement uncertainty is larger. In prac-
tice conglomerates of laboratories therefore focus on
minimizing bias amongst their measurement systems
and their imprecision.
Full method validation depends on the healt-
hcare and laboratory organizations
Healthcare laboratories are commonly organised
into larger laboratory conglomerates encompassing
several physical laboratories catering for diagnostic
services for a defined population. This caters for
important new opportunities for re-defining the
concept of a “laboratory” to encompass all laborato-
ries and measurement methods measuring the same
measurand for a population of patients.
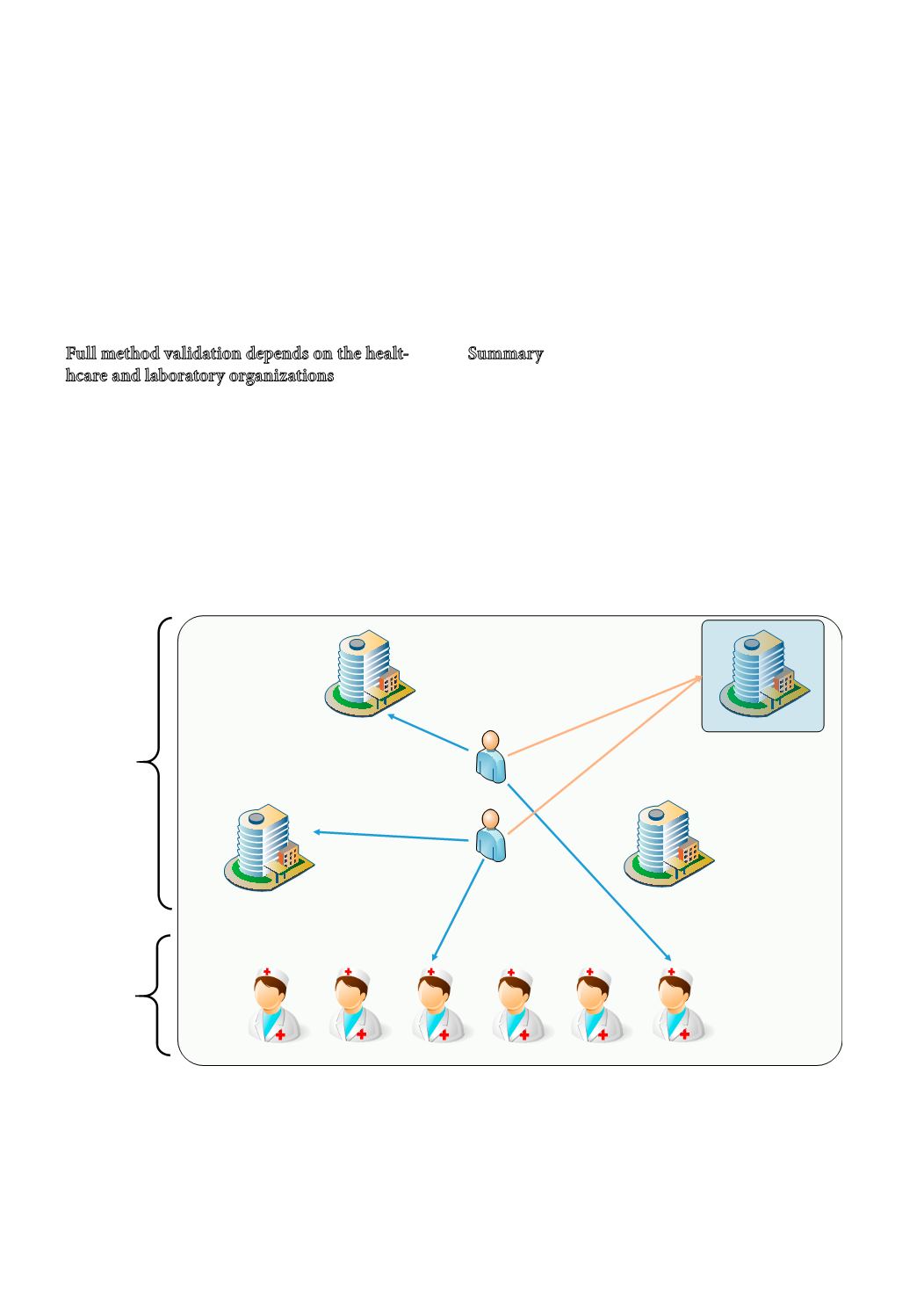
A single laboratory validation/verification is suf-
ficient if the same measurement system is always
used when analysing all samples from a population
of patients (situation “B” in figure 7). However, only
one measurement system used for measuring patient
samples is seldom the case in clinical chemistry.
Patients are commonly diagnosed and treatment
combined with monitoring initiated at large Univer-
sity hospitals to be continued at a smaller hospital and
one or two primary health-care physicians (Figure 7.)
Summary
Verification of measurement methods already valida-
ted by manufacturers is amongst the most common
activities in medical laboratories. A practical proce-
dure for verification using at least 20 natural patient
samples and measuring stable control samples during
one week is presented here, sufficient for most situa-
tions. Measurement systems are used in a plethora
of different situations where a sample for a patient
may encounter several measurement systems over
Figure 7
: The consequences of a conglomerate of laboratories catering for a patient population compared to a single labora-
tory. Hospital laboratory B caters for all samples for measuring a certain analyte. Validation of the single laboratory method
validation established by the manufacturer is in this case sufficient. In the case of other analytes measured in more than one
hospital laboratory and point of care, there are ample reasons to validate the fitness for purpose for diagnosing and monito-
ring treatment effects of the conglomerate of laboratories.
Page
16
of
20
Figure 7
: The consequenc s of conglomerate of laboratories catering for a patient
population compared to a single laboratory. Hospital laboratory B caters for all samples
for measuring a certain analyte. Validation of the single laboratory method validation
established by the manufacturer is in this case sufficient. In the case of other analytes
A
B
C
D
Large
labora-
tories
Point-
of-care
laborat
ories


