Osteocalcln concentration (pg par L)
figurationproducedanassay,
which could be used for
measurement of osteocalcin
20.------------------------------------------,
content in serum within 5
hours. According to our
strategy, this assay was
supposed to give a positive
result only against intact or
almost intact osteocalcin.
Thisapproachwas testedwith
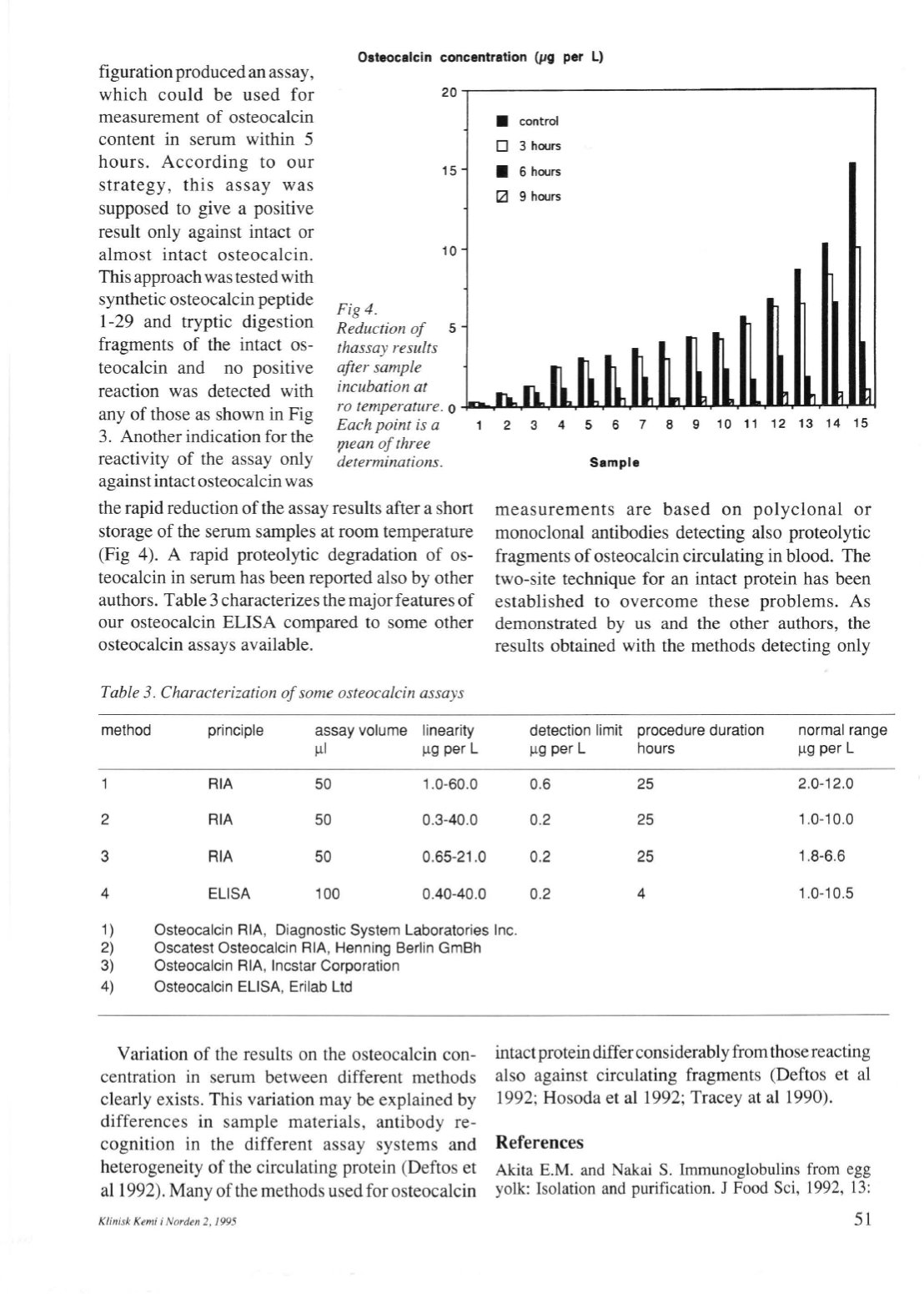
Fig 4.
Reduerian of
thassay resulls
after sample
incubation at
15
10
5
• centroi
D
3 hours
• 6 hours
[21
9 hours
syntheticosteocalcinpeptide
1-29 and tryptic digestion
fragments of the intact os–
teocalcin and no positive
reaction was detected with
any of those as shown in Fig
3. Another indication for the
reactivity of the assay only
againstintactosteocalcinwas
ro temperature.
o
~..,JI..Ib.,A.IIa.,II'-.U:!.,&&l.,lll.lliL,LIIl,IL.ai.,ILIIII..,a....,._..I..,IIJILI.,IL&I..,LI~
Eachpoint is a
91ean of three
determinations.
the rapid reductionof the assay results after a short
storage of the serum samples at room temperature
(Fig 4). A rapid proteolytic degradation of os–
teocalcin in serum has been reported also by other
authors. Table3characterizes themajorfeatures of
our osteocalcin ELISA compared to some other
osteocalcin assays available.
Table 3. Characterization ofsome osteocalcin assays
method
principle
assay volume linearity
111
11g per L
RIA
50
1.0-60.0
2
RIA
50
0.3-40.0
3
RIA
50
0.65-21.0
4
ELISA
100
0.40-40.0
2 3 4 5 6 7 8 9 10 11 12 13 14 15
Sample
measurements are based on polyclonal or
monoclonal antibodies detecting also proteolytic
fragments ofosteocalcincirculating inblood. The
two-site technique for an intact protein has been
established to overcome these problems. As
demonstrated by us and the other authors, the
results obtained with the methods detecting only
detection limit procedure duration
normal range
11g per L
hours
11g per
L
0.6
25
2.0-12.0
0.2
25
1.0-10.0
0.2
25
1.8-6.6
0.2
4
1.0-10.5
1)
Osteocalcin RIA, Diagnostic System Laboratories lnc.
2)
Oscatest Osteocalcin RIA, Henning Berlin GmBh
3)
Osteocalcin RIA, lncstar Corporation
4)
Osteocalcin ELISA, Erilab Ltd
Variation of the results on the osteocalcin con–
centration in serum between different methods
clearly exists. This variationmay be explained by
differences in sample materials, antibody re–
cognition in the different assay systems and
heterogeneity of the circulating protein (Deftos et
al1992).Manyofthemethods used for osteocalcin
Klinisk Kemi
i
Norden
2,
1995
intactproteindifferconsiderablyfrom thosereacting
also against circulating fragments (Deftos et al
1992; Hosoda et al 1992; Tracey at al 1990).
References
Akita E.M. and Nakai S. Immunoglobulins from egg
yolk: Isolation and purification. J Food Sci, 1992, 13:
51


