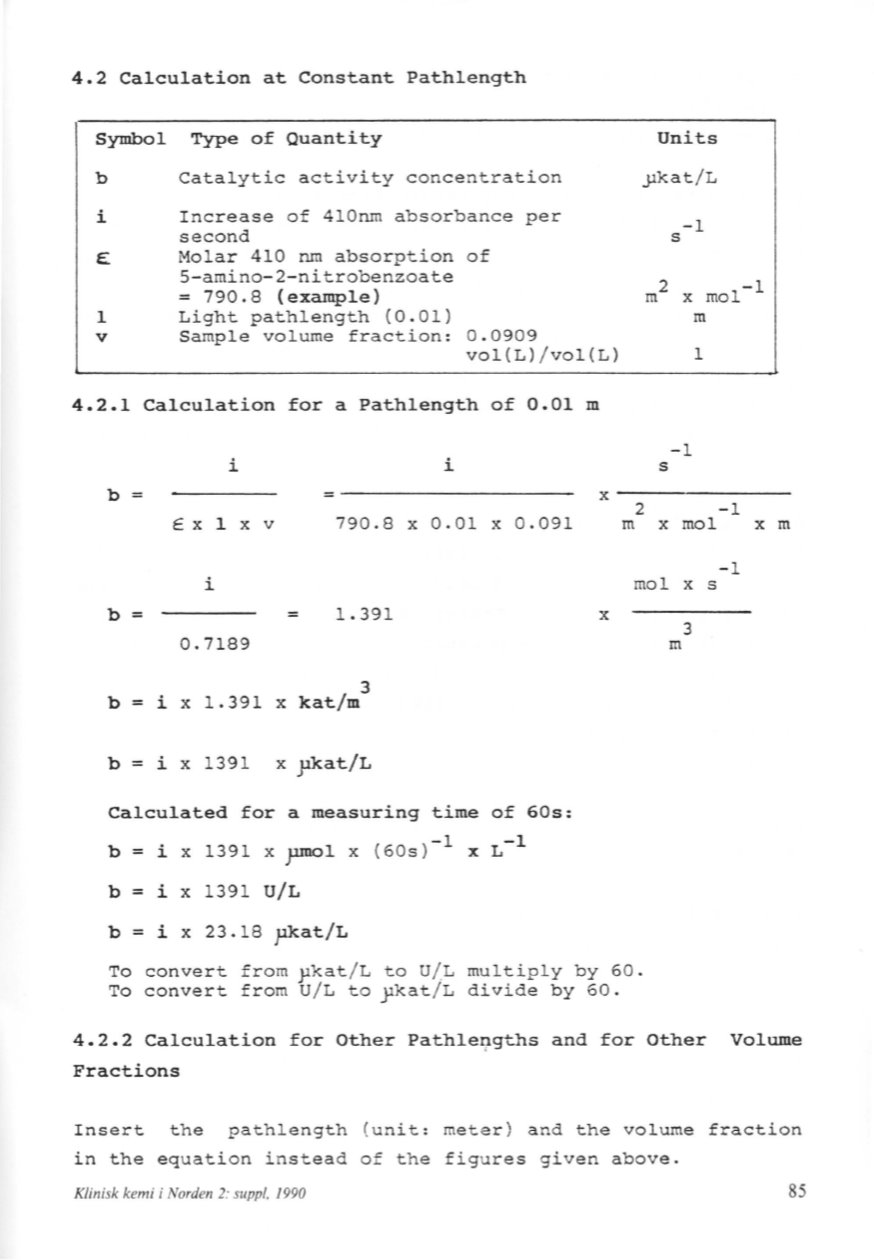
4.2 Calculation at eonstant Pathlength
Symbol Type of Quantity
b
Catalytic activity concentration
i
Increase of 410nm absorbance per
seeond
~
Molar 410 rum absorption of
5-amino-2-nitrobenzoate
=
790.8 (example)
l
Light pathlength (0.01)
v
Sample volume fraction: 0.0909
vol (L) /vol (L)
4.2.1 Calculation for a Pathlength of 0.01 m
i
i
b
=
=
Ex
l
x
v
790.8
x
0.01
x
0.091
i
b
=
=
l.
391
0.7189
k at/m
3
b
=
i
x l.
391
x
b
=
i x 1391 x pkat/L
Calculated for a measuring time of 60s:
b
=
i x 1391 x pmol x (60s)-l x L-l
b
=
i x 1391 U/L
b
=
i x 23.18 pkat/L
x
x
Units
ykat/L
-l
s
m2 x mol-l
m
l
-l
s
2
-l
m
x
mol
x
-l
mol
x
s
3
m
To convert from y.kat / L to U/ L multiply by 60.
To convert from U/L to ykat / L divide by 60.
m
4.2.2 Calculation for Other Pathle9gths and for Other Volume
Fractions
Insert the pathlength (unit: meter) and the volume fraction
in the equation instead of the figures given above.
Klinisk kemi
i
Norden 2: supp/, 1990
85


