PLOTSTRUCTURE of: a03301 .p2s September 30, 1993 13:35
PEPTIDESTRUCTURE
of:
a0330l.pirl Ck: 2554 , l
to :
49
Pl;GEHU - Osteocalcin - Human
10
20
lO
"
5.0 l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
'
l
l
l
l
l
l
l
KD Hydrophilici ty
-5.0
10.0
Surface Prob.
0 .0
--------==----==========~~--==~-----------===--~--~~====-----
1.2
Flexibility
0. 8
1.7
Jameson-Wolf
(Antiqenic Index)
-l.7
CF Turns
CF Alpha Helices
CF Beta Sheets
GOR Turns
GOR Alpha Helices
GOR Beta Sheets
Glycosyl. Si tes
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
1 l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
l
10
20
H
40
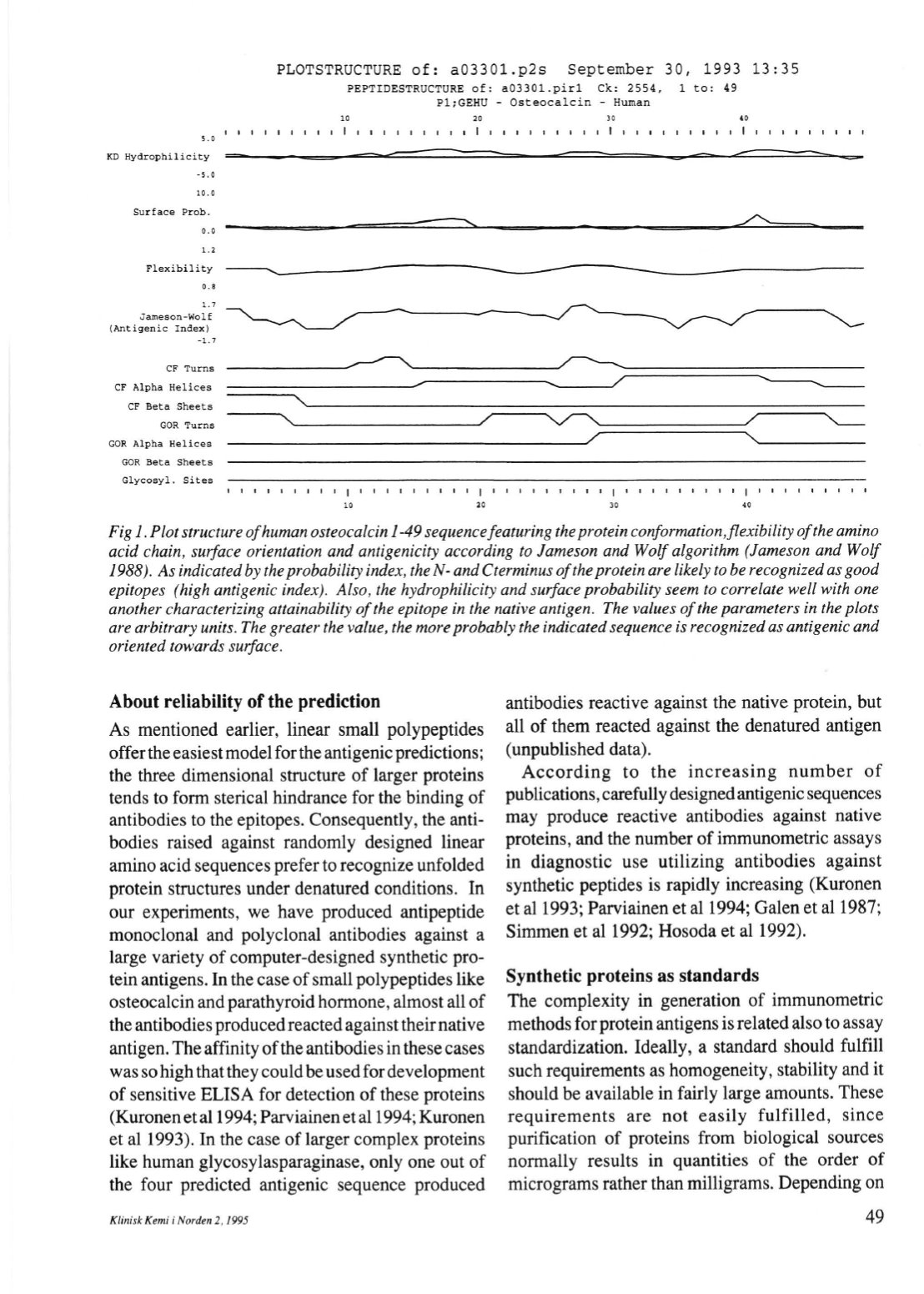
Figl .PIot structureofhumanosteocalcin1-49 sequencefeaturing theproteinconformation,flexibilityoftheamino
acid chain, surface orientation and antigenicity according to lameson andWolf algorithm (lameson andWolf
1988). As indicatedby theprohability index, theN- andCterminusoftheproteinare Iikely tobe recognizedasgood
epitopes (high antigenie index). A/so, the hydrophilicity and surface prohability seem to correlate weil with one
another characterizing attainability ofthe epitope in the native antigen. The values ofthe parameters in the plats
are arbitrary units. The greater the value, themoreprobably the indicatedsequence
is
recognizedasantigenieand
oriented towards surface.
About reliability of the prediction
As mentioned earlier, linear small polypeptides
offertheeasiestmodelfor theantigeniepredictions;
the three dimensional structure of larger proteins
tends to fonn sterical hindrance for the binding of
antibodies to the epitopes. Consequently, the anti–
bodies raised against randomly designed linear
amino acid sequencesprefer to recognizeunfolded
protein structures under denatured conditions. In
our experiments, we have produced antipeptide
monoclonal and polyclonal antibodies against a
large variety of computer-designed synthetic pro–
teinantigens. In thecaseofsmall polypeptides like
osteocalcinandparathyroidhonnone, almost all of
theantibodiesproducedreactedagainst theirnative
antigen.Theaffinityoftheantibodies in thesecases
wassohigh that theycouldbeusedfordevelopment
of sensitive ELISA for detection of these proteins
(Kuronenetall994; Parviainenetall994;Kuronen
et all993). In the case oflarger complex proteins
like human glycosylasparaginase, only one out of
the four predicted antigenie sequence produced
KliniskKemi
i
Norden
2,
1995
antibodies reactive against the native protein, but
all of them reacted against the denatured antigen
(unpublished data).
According to the increasing number of
publications,carefullydesignedantigeniesequences
may produce reactive antibodies against native
proteins, and the number of immunometric assays
in diagnostic use utilizing antibodies against
synthetic peptictes is rapidly increasing (Kuronen
et al1993; Parviainenet al1994; Galen et al1987;
Simmen et al 1992; Hosoda et al 1992).
Synthetic proteins as standards
The complexity in generation of immunometric
methods forproteinantigens is relatedalso toassay
standardization. Ideally, a standard should fulfill
such requirements as homogeneity, stability and it
should be available in fairly large amounts. These
requirements are not easily fulfilled, since
purification of proteins from biological sources
nonnally results in quantities of the order of
micrograms rather thanmilligrams. Dependingon
49


